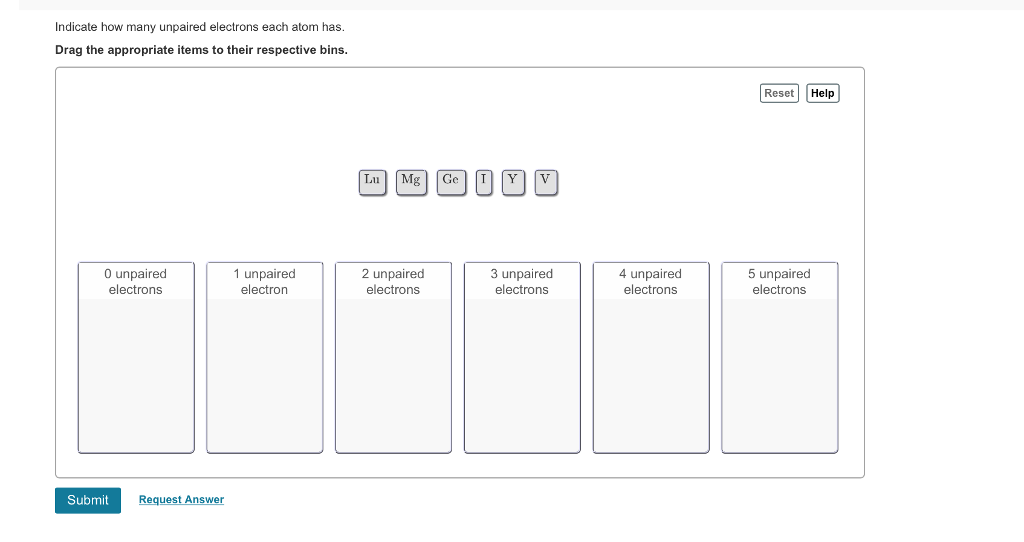
Indicate How Many Unpaired Electrons Each Atom Has.
12 How many electrons are in the p orbital of Ge. As Noble gases have no unpaired electrons the only unpaired electron in Gallium is the 4p1 electron so the answer is 1.
Solved Indicate How Many Unpaired Electrons Each Atom Has Chegg Com
1s2 2s2 2p6 3s2 3p6 4s1 3d5.
. How many unpaired electrons are in an atom. The following sets of quantum numbers listed in the order n ℓ mℓ and ms were written for the last electrons added to an atom. Drag the appropriate items to their respective bins Reset Help 0 unpaired electrons 1 unpaired 2 unpaired electrons 3 unpaired 4 unpaired electrons 5 unpaired electrons electron electrons Submit Request Answer.
9 Which atom in the ground state has 5 electrons in its outer level. Indicate how many unpaired electrons each atom has. No of unpaired electron 2 since p orbital can have maximum 6 electron c Chromium Cr.
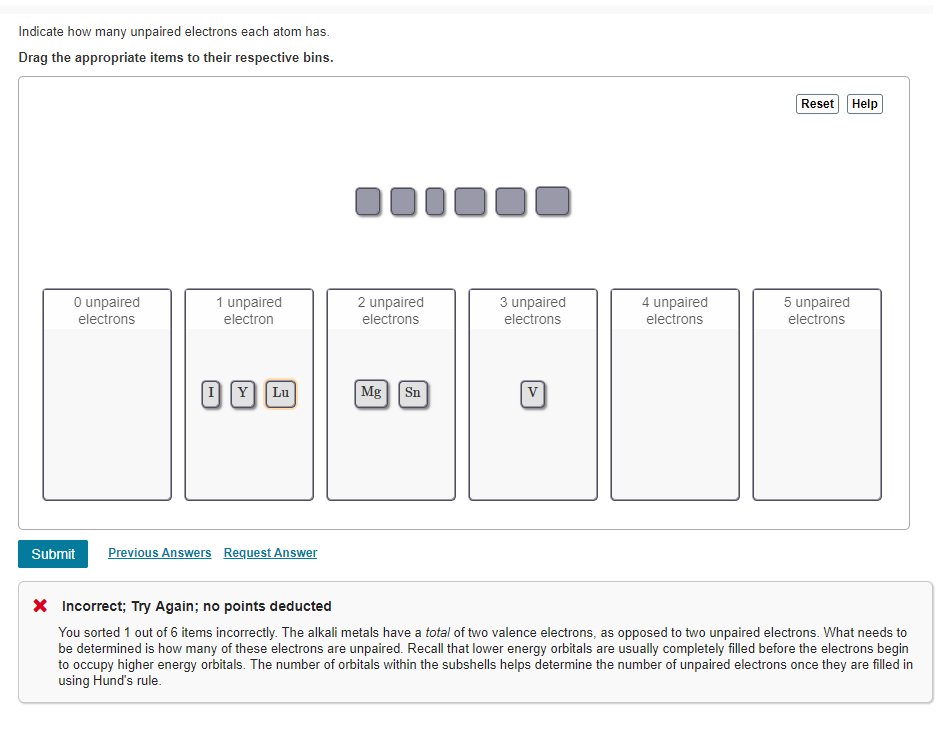
Chemistry questions and answers. Sn 3 unpaired electrons. In the element bromine Br there is only 1 unpaired electron.
Start your trial now. Indicate how many unpaired electrons each atom has. ResetHelp Ca Selected 0 unpaired electrons 1 unpaired electron 2 unpaired electrons 3 unpaired electrons 4 unpaired electrons 5 unpaired electrons.
Each atomic orbital of an atom specified by the three quantum numbers n l and m has a capacity to contain two electrons electron pair with opposite spins. Drag the appropriate items to their respective bins. Chemistry questions and answers.
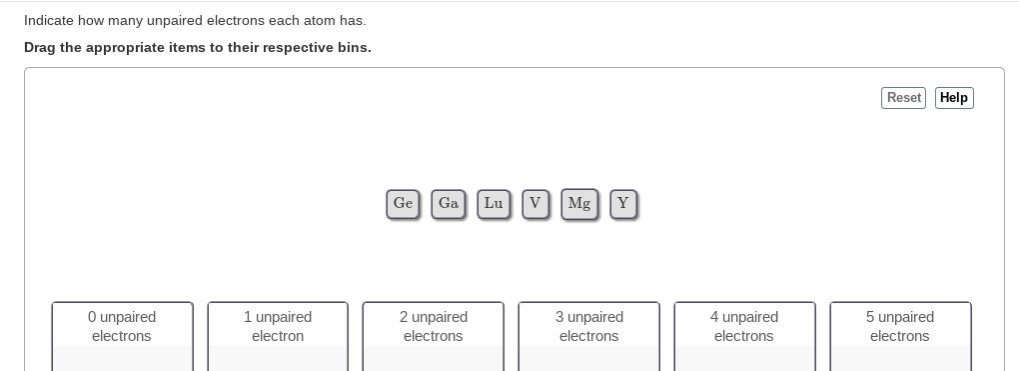
The condensed electronic configuration is. 6 How many core electrons are in Ge. Drag the appropriate items to their respective bins Reset Help 0 unpaired electrons 2 unpaired 3 unpaired 1 unpaired electron 5 unpaired electrons 4 unpaired electrons electrons electrons Lu.
Indicate how many unpaired electrons each atom has. It has 7 valence electrons so 3 pairs plus an unpairedelectron. 10 What element has the electron configuration 1s 2s 2p 3s 3p.
Ca 1 unpaired electron. Negative part of the addendum goes to the carbon atom having less number of. 8 Which atom in the ground state has two unpaired electrons.
Indicate how many unpaired electrons each atom has. Fluorine is in the 2nd Energy Level row of the Periodic Table and in the 5th column of the p block making the electron configuration 1s2 2s2 2p5 The outermost shell of the atom would have 7 electrons. Chemistry questions and answers.
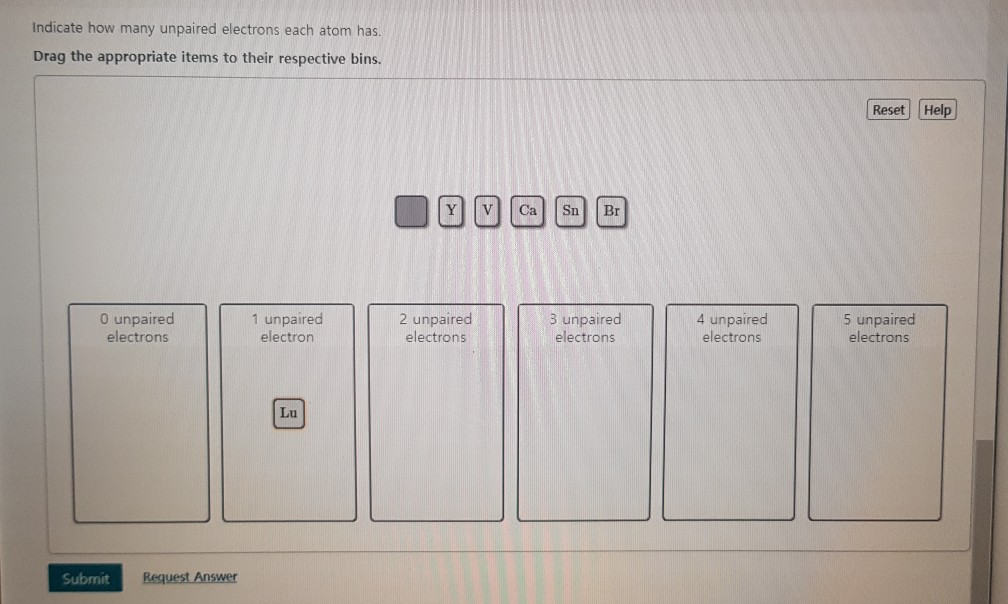
In chemistry an unpaired electron is an electron that occupies an orbital of an atom singly rather than as part of an electron pair. Indicate how many unpaired electrons each atom has. For Ca and Y for Ca i got Ar 4s2 but it got cuonted wrong 39948 results page 8 science.
2 Ca 2882 or 1s2 2s2 2p2 2p2 2p2 3s2 3p2 3p2 3p2 4s2 or Ar 4s2 - no unpaired electrons. 1s2 2s2 2p6 3s2 3p6 4s2 3d6. The radius of the electrons orbit in the Bohr model is 1323 nm.
N e3s2 N e 3 s 2. Textbf Electronic configuration Electronic configuration. A hydrogen atom has its electron in the n 5 level.
The number of unpaired electrons are 0. Drag the appropriate items to their respective bins. 11 How many neutrons does Ge have.
Indicate how many unpaired electrons each atom has. Mg Ge Br V. Reset Help 0 unpaired electrons 1 unpaired electron 2 unpaired electrons 3 unpaired electrons 4 unpaired electrons 5 unpaired electrons Previous Answers Request Answer X Incorrect.
Reset Help G L U ME Y O unpaired electrons 1 unpaired electron 2 unpaired electrons 3 unpaired electrons 4 unpaired electrons 5 unpaired electrons. Drag the appropriate items to their respective bins. Up to 256 cash back Designate unpaired electrons as UP arrows and keep all unpaired electrons and empty orbitals in the rightmost boxes of a subshell.
7 How many protons does Ge have. No of unpaired electrons 6 since 1 electron is to be added to 4s 5 electron to be added to 3d orbital. Indicate how many unpaired electrons each atom has Drag the appropriate items to their respective bins.
Fluorine has one unpaired electron in the ground state. Drag the appropriate items to their respective bins. See answer 1 Best Answer.
Identify which sets are valid and classify the others by the rule or principle that is violated. Textbf Unpaired electrons Unpaired electrons. Correct answer to the question Indicate how many unpaired electrons each atom has.
2Added explanationIn the outer orbitals of Pt Period VI 5d-blocktransition elements after the 4f-block-lanthanides there are two unpaired electronsthe first is the odd one in 4f17 or maybe. None 5 unpaired electrons. Fill in the last box in each atom with the number of unpaired electrons in the atom.
Reset Ca Selected 0 unpaired electrons 1 unpaired electron 2 unpaired electrons 3 unpaired electrons 4 unpaired electrons 5 unpaired electrons. Ar 4 s 2 3 d 10 4 p 2 4s23d 104p2 4 s 2 3 d 10 4 p 2. First week only 499.
V 4 unpaired electrons. Br Y Lu 2 unpaired electrons. Solution for Write the condensed electron configuration for the following atoms and indicate how many unpaired electrons each have.
See full answer below. Drag the appropriate items to their respective bins. No of unpaired electron 3 b Silicon Si.
Write the condensed electron configurations for the following atoms and indicate how many unpaired electrons each has. 1s2 2s2 2p6 3s2 3p2. An orbital is made up of 2 electrons and any orbitals left with only 1 electron is considered unpaired.
No points deducted You sorted 1 out of 6 items incorrectly.
Solved Indicate How Many Unpaired Electrons Each Atom Has Chegg Com

Solved Indicate How Many Unpaired Electrons Each Atom Has Chegg Com
Solved Indicate How Many Unpaired Electrons Each Atom Has Chegg Com
Comments
Post a Comment